Research
Synthesis of Compounds Having a Novel Bonding Containing Heavier Main Group Element
The periodic system of elements (the periodic table) defines elements with the same number of valence electrons as forming a family (element group) in which the individual elements and their compounds exhibit similar chemical and physical properties. Since chemistry has been developed on the basis of this periodic system, it is very important to consistently understand this system,
i.e.
, to compare the elements and their compounds from various viewpoints.
Meanwhile, low-coordinate species of main group elements of the second row such as carbenes, olefins, carbonyl compounds (ketones, aldehydes, esters, amides, etc.), aromatic compounds, and azo compounds played very important roles in organic chemistry. Although extensive studies have been devoted to these species not only from the physical organic points of view but also from the standpoints of synthetic chemistry and material science, the heavier element homologues of these low-coordinate species have only been postulated in many reactions as reactive intermediates and their chemistry has been undeveloped most probably due to their high reactivity and instability under ambient conditions.
At the beginning of the 20th century all attempts at synthesizing their heavier homologues proved unsuccessful, leading to cyclic oligomers or polymers containing only single covalent bonds. These findings and theoretical works lead to the view that "elements having a principal quantum number greater than two should not be able to form pp-pp bonds with themselves or with other elements", the so-called "classical double bond rule". However, this rule was disproved by the spectroscopic detection of a compound having a multiple P-C bond in 1961. Furthermore, the isolation of a stable phosphene (P=C) was reported in 1978, and in 1981 stable diphosphene (P=P), silene (Si=C), and disilene (Si=Si) were successively synthesized by taking advantage of steric protection.
For the stabilization of highly reactive compounds, there are two conceivable methodologies,
i.e.
, thermodynamic and kinetic stabilization. The former is defined as stabilization of the ground state by the mesomeric effect of neighboring heteroatoms, attachment of an electron-donating or -withdrawing substituents, or complexation with transition metals. The latter is stabilization resulting from raising the transition state by taking advantage of steric protection with bulky groups, which prevents oligomerization or reactions with other reagents such as oxygen and water. Kinetic stabilization is obviously superior to thermodynamic stabilization since the latter more largely perturbs the intrinsic nature of the species than the former.
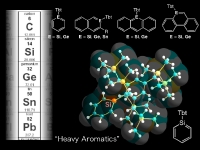
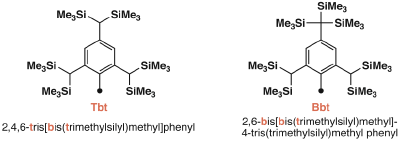
On the other hand, in the course of our study on the sterically congested molecules we have developed an extremely bulky aromatic substituent, 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl (denoted as Tbt hereafter) and 2,6-bis[bis(trimethylsilyl)methyl]-4-tris(trimethylsilyl)methyl phenyl (denoted as Bbt hereafter), which were found to be a very effective steric protection groups for a variety of reactive species of heavier main group element compounds.
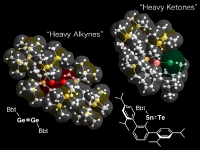
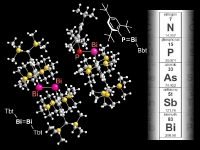
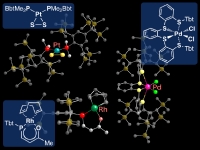
In our laboratory, we have synthesized a variety of unprecedented low-coordinate compounds of heavier main group elements as stable compounds by taking advantage of kinetic stabilization using a new type of steric protection groups, Tbt and Bbt. We have already succeeded in the isolation of various novel species such as group 14 element-group 16 element double-bond compounds [>M=X; M = Si, Ge, Sn, Pb; X = S, Se,Te], metallaaromatics, and doubly bonded compounds between group 15 elements [-M=M-, M = P, Sb, Bi]. In addition, we have also successfully synthesized novel species containing a small ring such as metallacyclopropabenzenes [>MC
6
H4
; M = Si, Ge] and platinum-dichalcogenido complexes [[Bbt(Me)2
P]2
PtX2
; X = S, Se]. The successful isolation and characterization of them provide us with a new research field and valuable information for the potential utility of heavier element compounds.